A Genomic Classifier for the Early Detection of Ectopic Pregnancy
June 30, 2021 | Download PDF
Did you know that nearly 16% of active component U.S. military members are women, and the majority are reproductive age? As the number of military servicewomen and their operational scope increase, it is important to understand and mitigate health issues that uniquely impact female servicemember readiness. Ectopic pregnancy accounts for 1-2% of all pregnancies in the United States and remains the leading cause of maternal death in the first trimester. An ectopic pregnancy can present with bleeding and/or pelvic pain. A serologically confirmed pregnancy that is clinically determined to be non-viable but cannot be reliably delineated as intrauterine or ectopic by ultrasound is defined as a non-viable pregnancy of unknown location (NV-PUL). Nearly 20% of women with a NV-PUL are ultimately found to have an ectopic pregnancy. In the absence of a biomarker to discern pregnancy location, patients are carefully followed with serial exams and labs to definitively resolve NV-PULs, and approximately half of patients require more than three clinic visits over a seven-day interval. This process risks loss to follow-up and delay in treatment in ectopic cases, which can result in tubal rupture and associated morbidity and mortality.
The absence of a reliable method for the early detection of ectopic pregnancy has important readiness implications for military servicewomen. Not only does the current PUL management algorithm translate to lost time from work/training, but the psychological stress associated with the uncertainty during this interval may negatively impact performance. Early detection of ectopic pregnancy allows intervention at a time when medical management has a better chance of success, thereby obviating the need for surgery, surgical risks and more lengthy recovery timeframes. A biomarker or classifier is a molecular analyte or panel of analytes used to diagnose a particular disease condition. Actuarial studies reveal that a biomarker for ectopic pregnancy would save approximately $1880 in healthcare costs per patient. By reducing the number of visits required to diagnose pregnancy location, a classifier improves overall access to care. Most importantly, a classifier that allows the early detection of ectopic pregnancy reduces the likelihood of tubal rupture associated with diagnostic delay, thereby improving patient safety and military readiness.
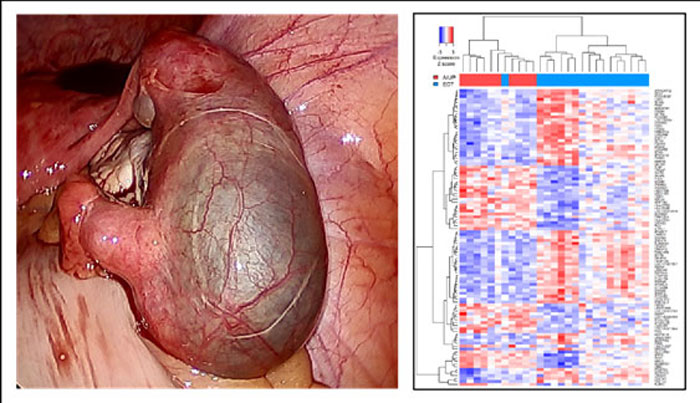
The past decade has witnessed remarkable advances in high throughput molecular techniques and bioinformatics approaches culminating in more personalized diagnostics and treatments. At Madigan Army Medical Center, Precision Medicine Research is facilitated by the Department of Clinical Investigation’s (DCI) core laboratory that includes biobank, proteogenomic and bioinformatics services. A team from the Department of Obstetrics and Gynecology led by Principal Investigator, Dr. (COL retired) Greg Chow, hypothesized that detectable molecular differences exist in intrauterine samples from women with ectopic pregnancy compared to women with miscarriage, and the most reliably detected differences could be included in a molecular classifier to delineate pregnancy location.
In an IRB-approved global gene expression study involving samples from 65 women with ectopic pregnancy or miscarriage, an intrauterine genomic classifier was developed by the Madigan team. The five most reliably differentially expressed genes were combined to formulate a classifier for pregnancy location. In this AMTI-funded demonstration, the classifier was independently tested and found to be 91% (42/46) accurate in delineating pregnancy location as intrauterine or ectopic. Importantly, the classifier was translated to an n-counter (Nanostring) detection strategy to abbreviate turn-around-time to less than 24 hours. The technology was filed for full utility patent in March 2019. Co-inventor and Madigan Reproductive Endocrinology and Infertility (REI) Fellow, MAJ Jessica Lentscher, presented the results at the 2019 meeting of the Society for Reproductive Investigation, winning the Best Investigator Award. Findings were published as a peer-reviewed manuscript in Fertility and Sterility, a leading journal in Women’s Health.
“Funding from the AMTI program made this go – this is a great example of military GME partnering with DCI to address a military-relevant gap with patented innovation,” said Dr. (COL retired) Rick Burney, Chief of DCI and a co-investigator/co-inventor. “Given the cost and scale, the refinement and demonstration of this molecular classifier would not have been possible without AMTI investment.” To build on these successes, the team plans to analyze proteogenomic signatures of surgically removed tubal pregnancies toward identifying other potential biomarker/classifier candidates.
For additional information on this AMTI funded project, please contact Ms. Holly Pavliscsak at holly.h.pavliscsak.ctr@mail.mil.
This article was published in the January 2022 issue of the TATRC Times.