Commander’s Corner: A Message from COL Jeremy Pamplin
Friday, August 30, 2024 | Download PDF

Hello Friends and Colleagues! As we’ve marched through this year, Team TATRC continues to amaze me on a regular basis. There’s been a palpable change over the last few months with the ability to get things done and I’m really proud of that. It’s impressive to witness.
Since my last update in the early Spring, TATRC has completed its first official “Sprint” in our new mission oriented, programmatic research model. Each of TATRC’s functional areas have been driving hard toward our mission to automate casualty care with laser beam focus on our first material product solution, “AutoDoc.”
The Medical Modeling Simulation, Information, and Visualization (MMSIV) team wrapped up the first sprint of “AutoDoc” focused on establishing external partners to collaborate on data collection and worked hard to get all the necessary protocols and agreements into place so that we could scale data collection during our summary sprint. As part of that scale up, the MMSIV team has refined and validated our data collection processes and Standard Operating Procedures (SOPs) so that our partners could easily follow them.
MMSIV’s focus for Sprint 2 is “filling the data bucket” and demonstrating value through value metrics (Figure 1). The team has successfully collected data with six partner locations (the Medical Center of Excellence, Defense Medical Readiness Training Institute, and 59th Medical Wing at Joint Base San Antonio, the Joint Special Operations Medical Training Center at Fort Liberty, 4/166 Regional Training Institute at Fort Indian Town Gap, and Ragged Edge Solutions) and performed weekly data collection within the TATRC NEXUS lab. We are well on our way to achieve this sprint’s goal to collect 2,500 procedures (individual tasks) and 250 simulations (10-20 min casualty care scenarios) to baseline performance data and using the TATRC “AutoDoc” sensor suite (camera, microphone, and accelerometers).
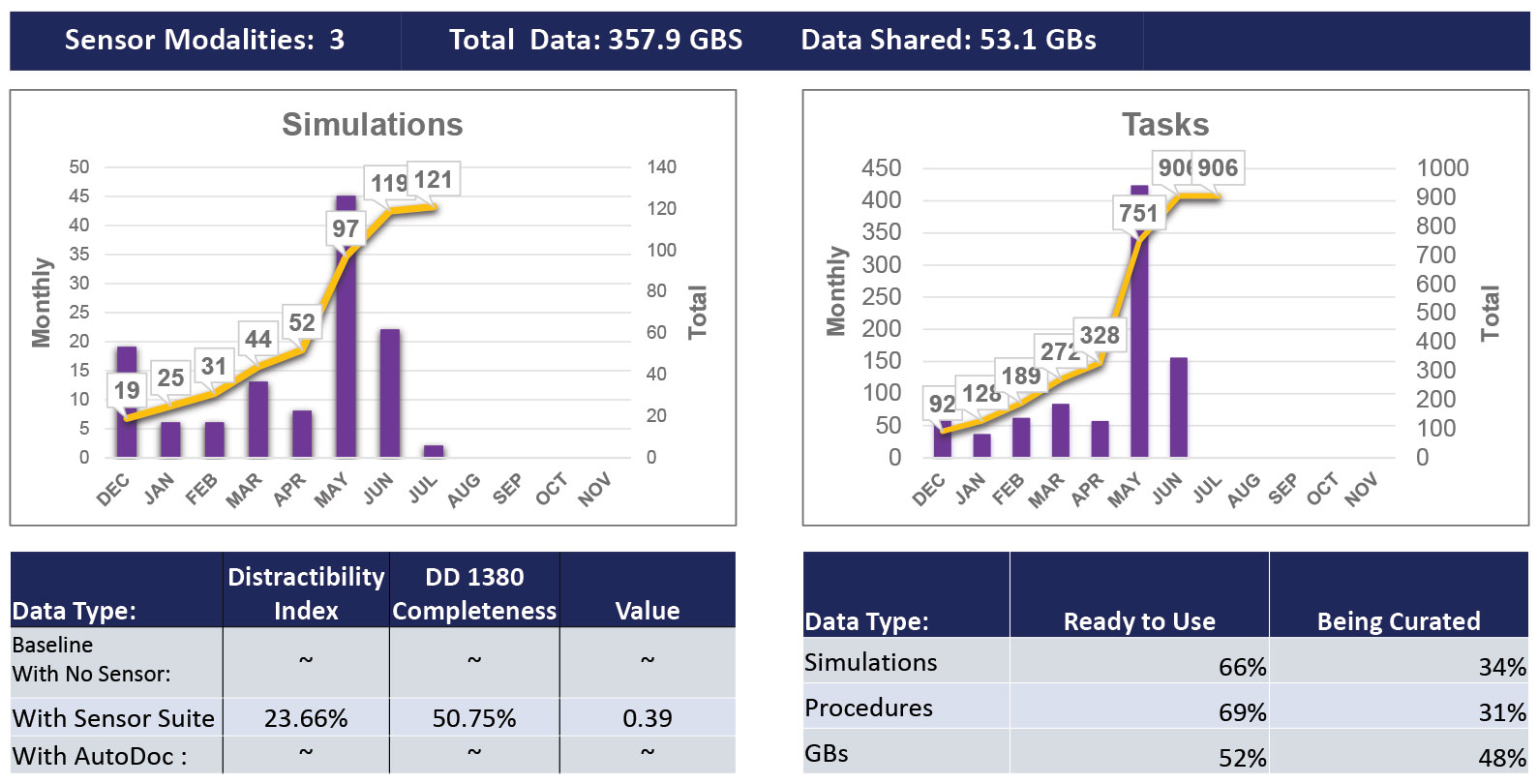 Figure 1. TATRC AutoDoc Project Value Metrics. This image shows that we have collected nearly 360GB of data using 3 sensors worn by research participants. This represents 121 simulations (10-20 min casualty management scenarios), and 906 individual tasks (like tourniquet placement or IV placement). Finally, the bottom tables show the distractibility index (DI = time documenting + time using sensors divided by total scenario time) and the DD Form 1380 completeness (total accurate completed sections divided by expected sections). The “Value” metric is a summary of DI and DD 1380 completeness ((1-DI)*DD 1380 completeness). Finally, we show the percentage of data that has been collected (ready to use) vs. curated (labeled with task start and stop times). Finally, we have shared just over 50GB of data with TATRC research partners for initial data models.
Figure 1. TATRC AutoDoc Project Value Metrics. This image shows that we have collected nearly 360GB of data using 3 sensors worn by research participants. This represents 121 simulations (10-20 min casualty management scenarios), and 906 individual tasks (like tourniquet placement or IV placement). Finally, the bottom tables show the distractibility index (DI = time documenting + time using sensors divided by total scenario time) and the DD Form 1380 completeness (total accurate completed sections divided by expected sections). The “Value” metric is a summary of DI and DD 1380 completeness ((1-DI)*DD 1380 completeness). Finally, we show the percentage of data that has been collected (ready to use) vs. curated (labeled with task start and stop times). Finally, we have shared just over 50GB of data with TATRC research partners for initial data models.
Indeed, we’ve learned a lot about average time-on-task for individual procedures (like, it takes on average, less than a minute to place a tourniquet or perform a needle decompression, but nearly two minutes to pack a junctional wound, three minutes to start an IV, and nine minutes to place a good pressure dressing). Furthermore, with this data, we are able to provide medics feedback about their performance compared to the mean on various casualty care models (Figures 2 & 3), and our data-set continues to grow, increasing our ability to define expectations and determine expected performance on average, individual performance, and subsequently, more precise training requirements.
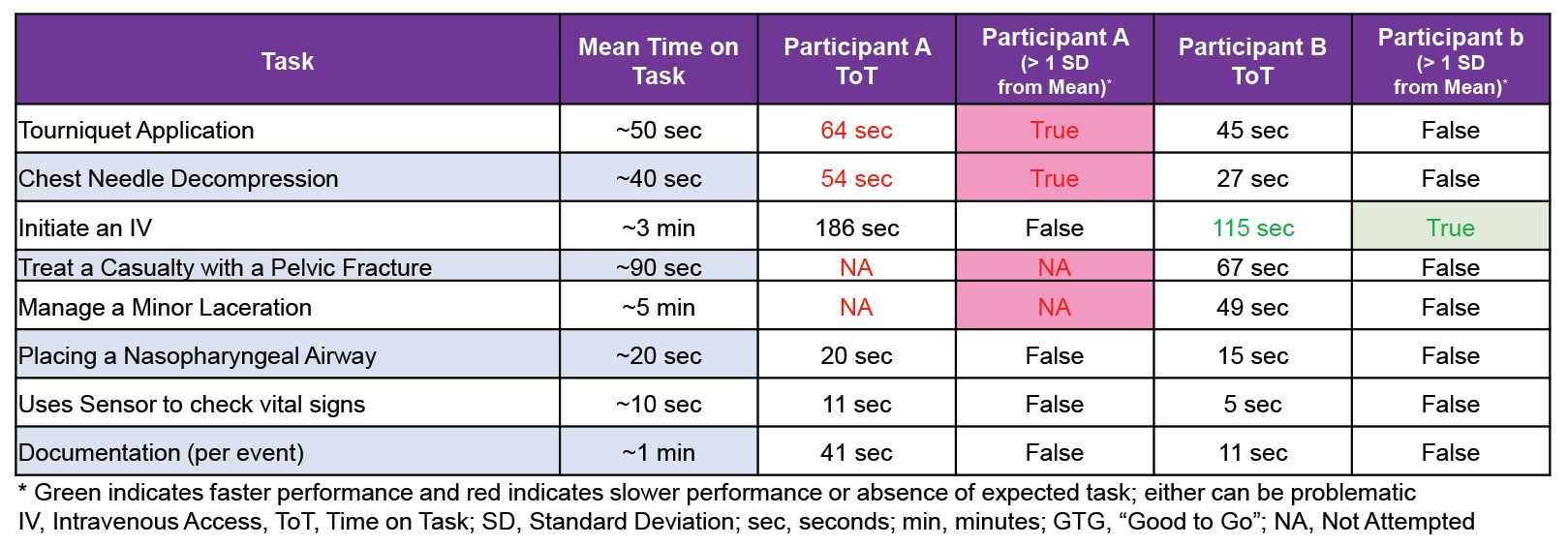 Figure 1. TATRC AutoDoc Project Value Metrics. This image shows that we have collected nearly 360GB of data using 3 sensors worn by research participants. This represents 121 simulations (10-20 min casualty management scenarios), and 906 individual tasks (like tourniquet placement or IV placement). Finally, the bottom tables show the distractibility index (DI = time documenting + time using sensors divided by total scenario time) and the DD Form 1380 completeness (total accurate completed sections divided by expected sections). The “Value” metric is a summary of DI and DD 1380 completeness ((1-DI)*DD 1380 completeness). Finally, we show the percentage of data that has been collected (ready to use) vs. curated (labeled with task start and stop times). Finally, we have shared just over 50GB of data with TATRC research partners for initial data models.
Figure 1. TATRC AutoDoc Project Value Metrics. This image shows that we have collected nearly 360GB of data using 3 sensors worn by research participants. This represents 121 simulations (10-20 min casualty management scenarios), and 906 individual tasks (like tourniquet placement or IV placement). Finally, the bottom tables show the distractibility index (DI = time documenting + time using sensors divided by total scenario time) and the DD Form 1380 completeness (total accurate completed sections divided by expected sections). The “Value” metric is a summary of DI and DD 1380 completeness ((1-DI)*DD 1380 completeness). Finally, we show the percentage of data that has been collected (ready to use) vs. curated (labeled with task start and stop times). Finally, we have shared just over 50GB of data with TATRC research partners for initial data models.
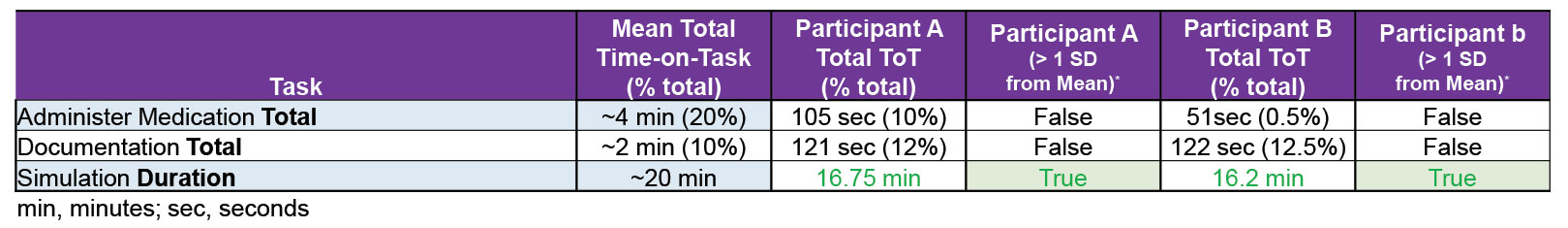 Figure 2. Example showing performance characteristics across four (4) casualty models and individual participants’ comparison to the mean.
Figure 2. Example showing performance characteristics across four (4) casualty models and individual participants’ comparison to the mean.
Meanwhile, the Medical Robotics and Autonomous Systems (MedRAS) team finished its first Sprint by identifying and delivering the TATRC AutoDoc prototype sensor suite! Data from this type of sensor suite is being used to create AI models that identify casualty status (injury patterns), caregiver actions (like a needle decompression or tourniquet placements), and resources consumed (like a needle or a tourniquet), and produce discrete data elements that can populate the appropriate sections of a Form DD 1380 (Figure 4). In the first sprint, development started on a tablet-based centralized point of aggregation to collect all incoming sensor data using a “PoTAg” (a Point of Treatment Aggregator) and also a web-based aggregation point to compile all sensor and related scenario metadata (the “IDA” or Intermediate Data Aggregator).
 Figure 4. Example Video footage with TATRC partner Arete’s machine vision model identifying a Right Arm Amputation and automatically placing an indicator on a DD Form 1380 (tactical combat casualty care card).
Figure 4. Example Video footage with TATRC partner Arete’s machine vision model identifying a Right Arm Amputation and automatically placing an indicator on a DD Form 1380 (tactical combat casualty care card).
In Sprint 2, the MedRAS team is focused on two new important tasks: software development and algorithm maturation. Development has continued to further refine PoTAg and IDA to increase the useability of the data collected. Development has also focused on expanding the pipeline of data flow from the source of collection to a cloud-based storage solution, including a multi-directional connection between PoTAg, Medic CDSS, and BATDOK. The second focus is working with our intramural algorithm partners to expand existing algorithms and incorporate them into our sensor suite to provide DD 1380 outputs. By the end of sprint 2, MedRAS plans to deliver a proof-of-concept prototype showing a subsection of the inputs required for a DD 1380 automatically populated from algorithms processing sensor data in real-time!
Finally, our Science & Technology, Innovation Management & Synchronization (STIMS) team completed Sprint 1 by facilitating agreements for MMSIV’s six new data collection partners and Medical Technology Enterprise Consortium (MTEC) contract awards to Ragged Edge Solutions and MITRE, a federally funded research and development center (FFRDC) to continue managing the Device Interoperability and Autonomy Coordinating Center (DIACC), which you’ll read more about inside this edition of our newsletter. STIMS finished strong by closing out three legacy projects (FOXTROT and two Monkeypox projects) and enabled a contract award to 3M for a CSI involving Network Enabled-Digital Wound Dressing (fascinating stuff!).
The STIMS Sprint 2 aim is to identify a clinical partner(s) and prepare protocols with them to study TATRC technologies in clinical settings with real patients. Not only have our teams made great strides in our research sprints, but collaborations with our external partners have also seen significant progress.
On 29 May, TATRC participated in the 3rd Quarterly Data Nexus symposium, a collaborative meeting that brings together stakeholders who are interested in a common data space across research, training, and real casualty care domains, a space we collectively called the “Data Nexus.” This collaborative group seeks to build consensus amongst experts in the areas of data collection models and methods, storage, use, and priorities of work. The hybrid event hosted 79 participants both virtually and in person at the SFC Paul Ray Smith Simulation and Training Technology Center (STTC) in Orlando, FL. Key participants included the Program Executive Office (PEO) for Simulation, Training and Instrumentation, PEO Defense Healthcare Management Systems, Joint Trauma System, Combat Casualty Care Research Program, Combat Capabilities Development Command, DHA, United Kingdom SMO for Telemed Army, and our close partner, the MIT Lincoln Laboratory. The Data Nexus collaboration continues to grow and gain momentum. We look forward to seeing everyone at the 4th symposium that will occur at the conclusion of MHSRS ‘24 in Orlando.
A few other noteworthy events are worth highlighting since our last edition of the TATRC Times.
At the beginning of June, TATRC participated in the first of three DARPA Triage Challenge (DTC) https://triagechallenge.darpa.mil/ workshops at the Guardian Centers Facility in Perry, GA. TATRC plays a key role in the DTC as an Independent Verification and Validation (IV&V) partner that creates opportunities for DTC performers to test their remote sensing solutions and inform their preliminary digital and AI triage models. This workshop was the culmination of over a year’s worth of effort and included increasing realism for casualty simulations by utilizing live actors with more realistic moulage. The DTC collaboration is essential for advancing AI and robotic enabled triage solutions. We’re pleased and appreciative to be a key partner for DARPA in this effort.
Not only did the MedRAS team participate in DTC, but they also held successful flight tests of the Remote Patient Management System (RPMS). In collaboration with our partners at the U.S. Army Aeromedical Research Laboratory (USAARL), the team flew the RPMS platform onboard a UH-60M helicopter at Frederick Municipal Airport. The RPMS is an integrated medical device platform that supports remote control and monitoring of the Thornhill MOVES SLC ventilator with physiological sensors, the NeuroWave Sciences infusion pump, and the DocBox data integrator and control software. The testing explored remote management capabilities and tactical network performance in a simulated air-MEDEVAC environment. This experimental data provided a tactical network characterization that will be used to optimize the RPMS system for future telemedicine applications, and, in the future, integration with autonomous controllers to facilitate fully autonomous casualty transport in low-bandwidth and contested environments.
 TATRC Commander, COL Jeremy Pamplin and Science Director, Matt Quinn served as panelists at the inaugural meeting of the Massachusetts General Hospital Center for Smart and Autonomous Medical Systems (SaAMS), part of the Medical Device Plug and Play Program (MDPnP).
TATRC Commander, COL Jeremy Pamplin and Science Director, Matt Quinn served as panelists at the inaugural meeting of the Massachusetts General Hospital Center for Smart and Autonomous Medical Systems (SaAMS), part of the Medical Device Plug and Play Program (MDPnP).In June, our Science Director, Matt Quinn, and I served as panelists at the inaugural meeting of the Massachusetts General Hospital Center for Smart and Autonomous Medical Systems (SaAMS), part of the Medical Device Plug and Play Program (MDPnP). The SaAMS is an FDA-endorsed, Joint Warfighter Sponsored Collaborative Community that focuses on advancing systems that securely connect heterogeneous medical devices, sensors, and algorithms into autonomous and semi-autonomous systems. Dr. Julian Goldman, the SaAMS Collaborative Community lead, was instrumental in TATRC’s work on the Remote Patient Management System during COVID, Project Convergence Capstone, and the FDA’s recent Guidance on Physiologic Closed Loop Control. (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/technical-considerations-medical-devices-physiologic-closed-loop-control-technology). TATRC has a CRADA with the MDPnP Program and SaAMS Collaborative to advance autonomous systems.
Another proud moment from this past quarter included the recognition at the Annual FORUM Innovation Awards of two TATRC project: Project VISTA (Vision and Intelligence Systems for Medical Teaming Applications) and the aforementioned RPMS. These projects were nominated by Government and Industry professionals and selected by a committee of their peers, for showcasing breakthrough innovations that improve and advance each agency’s mission. Mr. Matt Quinn, Ethan Quist, and Zack Buono were in attendance to accept the awards. Congratulations to the team and all the hard work you put in on each of these projects!
 Two TATRC project: Project VISTA (Vision and Intelligence Systems for Medical Teaming Applications) and RPMS (Remote Patient Management System) were recognized at the Annual FORUM Innovation Awards.
Two TATRC project: Project VISTA (Vision and Intelligence Systems for Medical Teaming Applications) and RPMS (Remote Patient Management System) were recognized at the Annual FORUM Innovation Awards.
I would like to take this opportunity to express my gratitude to every one of the TATRC Staff for the tremendous support and hard work that they have provided. The dedication and effort that you have all put into your work are truly appreciated: we couldn’t achieve these goals without your valuable contributions. Thank you for your commitment to excellence and for being an integral part of our team, your hard work and dedication does not go unnoticed.
As you can see, there’s no rest for the weary… or innovators for that matter! I continue to be amazed by the work our team does every day and I’m incredibly proud to work alongside these dedicated professionals while they relentlessly pursue ways to enhance the care of our casualties. #FindAWay!
This article was published in the August 2024 issue of the TATRC Times.