First DoD Medical Capability Developed Using Artificial Intelligence Receives FDA Clearance
Friday, August 30, 2024 | Download PDF
Did you know that hemorrhage is the leading cause of preventable death on the battlefield, where more than 90% of combat casualties die before ever reaching a medical treatment facility? However, it is challenging to identify trauma casualties at risk for uncontrolled bleeding at the point of injury from those who are injured but may not be at risk for hemorrhage.
The U.S. Army Medical Research and Development Command’s (MRDC) Biotechnology High Performance Computing Software Applications Institute (BHSAI) recently obtained FDA clearance of the Automated Processing of the Physiological Registry for Assessment of Injury Severity-Hemorrhage Risk Index (APPRAISE-HRI) software as a medical device.
APPRAISE-HRI is an artificial intelligence enabled decision-support mobile health application intended to help military health care providers screen U.S. Service Members for hemorrhage risk after a physically traumatic event and stratify casualties who might need immediate attention and emergency evacuation from those who are injured but may not be at risk for hemorrhage.
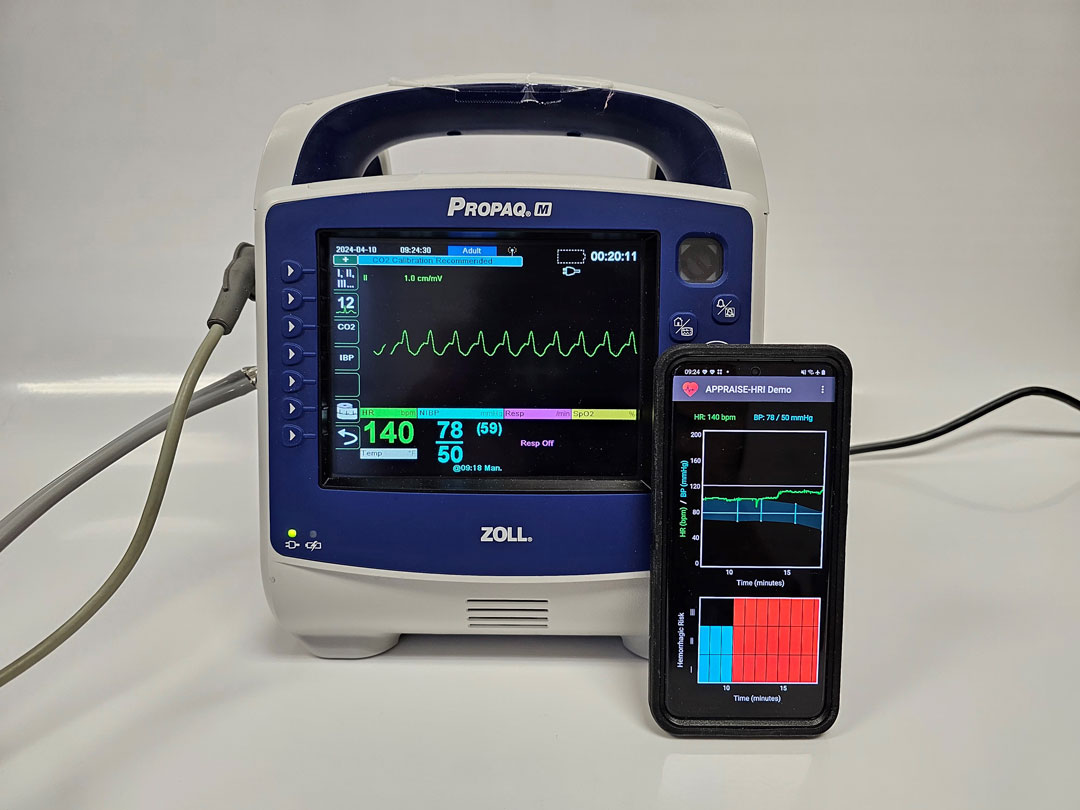
The APPRAISE-HRI consists of a commercial vital-sign monitor and an AI app running on an Android smartphone. The monitor continuously collects routine heart rate and blood pressure data from the casualty and wirelessly transmits the data to the smartphone, where the AI app analyzes patterns in the vital signs and provides a hemorrhage risk score.
To train the AI algorithm, MRDC scientists conducted three clinical studies to collect real-world vital-sign data from about 2,000 civilian trauma casualties either during ground- and air-ambulance transport from the point of injury to a receiving hospital or in the Emergency Department. To obtain the FDA 510(k) clearance, MRDC blindly and independently assessed the AI algorithm using vital-sign data collected from an additional 6,000 trauma patients at nine different sites. The independent assessment showed that the APPRAISE-HRI was highly effective in stratifying the likelihood that a trauma patient experienced hemorrhage within the first 10 minutes of patient monitoring. The DoD holds three U.S. patents on APPRAISE-HRI.
The APPRAISE-HRI offers the unique capability to automatically integrate and extract information from standard vital signs in a systematic and reproducible manner, to improve situational awareness and help medics identify the most severely injured casualties. The device’s risk score is easy to interpret and the use of standard vital signs will minimize training and facilitate dissemination.
APPRAISE-HRI is not intended to diagnose or direct treatment. Rather, it is intended to serve as a risk assessment tool and to provide situational awareness and inform clinical management of potentially hemorrhagic casualties by identifying those at the greatest risk of hemorrhage.
This research effort was sponsored by the U.S. Army Combat Casualty Care Research Program, which funded the research, and the U.S. Army Medical Materiel Development Activity (USAMMDA), which provided funding for the regulatory activities with the FDA, with support from the Defense Health Program. This important milestone is the result of an interagency agreement sponsored by the Warfighter Expeditionary Medicine and Treatment Project Management Office, part of USAMMDA. The agreement was established in support of the Defense Health Agency's Advanced Medical Monitor Family of Systems program, which aims to provide far-forward medical monitoring capability to the joint services.
The MRDC Office of Regulated Activities provided support with the interactions between the FDA and the BHSAI. The Medical Technology Transfer Office is seeking to license this technology, for which the U.S. Army holds three U.S. patents, with interested commercial partners.
For more information on this BHSAI initiative, please contact Dr. Jaques Reifman at: jaques.reifman.civ@health.mil.
This article was published in the August 2024 issue of the TATRC Times.