AMTI Project Spotlight: Optimizing REBOA for the Austere Combat Environment Using the COMPASS® Portable Pressure Monitor
March 31, 2021 | Download PDF
Although there have been great advances in the care of Service Members wounded in combat and other operational environments, bleeding remains a common cause of death. Among deaths that have been determined to be “potentially preventable,” hemorrhage has been identified as the primary cause in 80-90% of cases. Of these, approximately half are due to bleeding within the chest or abdominal body cavities. Until recently, there have been no available options to treat and stabilize patients with this type of bleeding outside of a fully staffed hospital operating room. A relatively new technique called the Resuscitative Endovascular Balloon Occlusion of the Aorta, or REBOA, has been developed and can be used to slow or stop hemorrhage and stabilize a wounded Service Member until they can get to a facility with surgeons and an operating room. This procedure involves inserting a catheter with a balloon tip through the groin and into the aorta, and then inflation of the balloon to stop all blood flow to the injured area.
However, one of the limitations of REBOA is that it requires the availability of an advanced patient monitoring device and equipment that is connected to the REBOA catheter to continuously monitor the patient’s blood pressure and other vital signs. This makes it difficult or impractical to utilize in many austere battlefield settings. The research team sought to evaluate and validate the utility of a new minitiarized and handheld digital blood pressure device called a COMPASS® (Mirador Biomedical, Seattle, WA) for guiding REBOA therapy and potentially eliminating the need for the usual advanced (and non-portable) pressure monitoring equipment. This project involved a series of validation exercises utilizing a realistic animal model of severe abdominal injury with massive bleeding, and direct comparison of the COMPASS device to the standard bulkier monitoring systems. Of note, this project also represented a new and highly productive and collaborative inter-service effort between investigators from the U.S. Army at Madigan Army Medical Center and the U.S. Navy at Naval Medical Center San Diego.
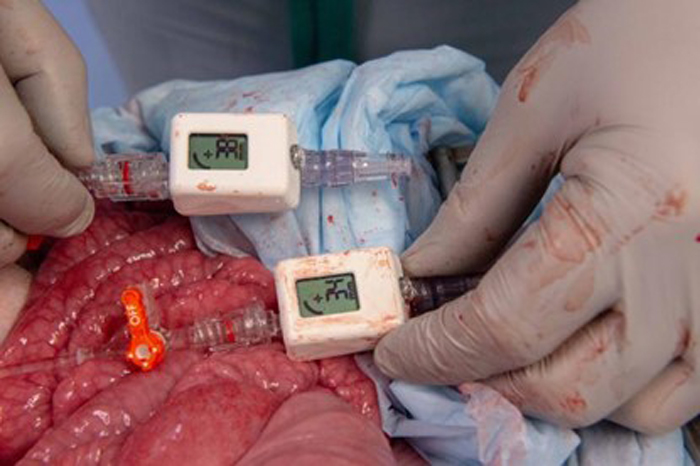
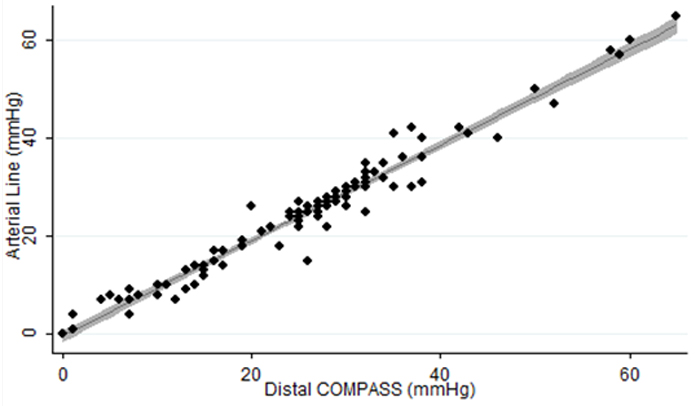
Results from the initial series of validation tests of the COMPASS device in uninjured healthy animals demonstrated that the device is highly accurate and reliable compared to standard blood pressure monitoring systems. Subsequent validation tests then examined the accuracy and reliability of the device under a number of “real-world” conditions including animals with massive hemorrhage, and with monitoring the response to treatment with REBOA. In these series of tests, the COMPASS device again demonstrated a high degree of accuracy and reliability in comparison to use of the larger and more expensive monitoring systems. In a final set of validation tests, the ability of a lone medical provider to treat animals with massive hemorrhage using only the COMPASS device to initiate and then adjust treatment with a REBOA catheter was evaluated. These tests confirmed that a medical provider could successfully initiate and then adjust REBOA therapy using only the minitiarized COMPASS device and without the need for the larger and bulkier blood pressure monitoring systems.
This project and the resulting data speak directly to multiple pillars of the Advanced Medical Technology Initiative (AMTI)’s Program. The most obvious pillar is readiness and preparedness of the medical forces for forward deployment and providing high quality and effective care to patients with massive bleeding. Use of these devices in combination have the potential to greatly improve the ability of austere forward surgical or resuscitative medical units to immediately intervene and stabilize severely wounded Service Members. The project also speaks to the pillars of reducing cost. The use of the studied devices could greatly simplify the required equipment that is needed to implement a REBOA program in the forward deployed environment, including eliminating the need for the bulky and expensive patient monitoring systems that are currently required. The effective use of REBOA, and utilization of the COMPASS device to accurately guide therapy, may also result in decreased costs through reducing the need for multiple blood transfusions or other interventions that are required when there is no ability for early control of massive bleeding. This work could also greatly impact the pillar of quality, by providing what would currently be the only effective forward capability for control of torso hemorrhage outside of the operating room environment.
The work from this project has already had significant impact on the Military Health System, and will continue to have impact as additional data analyses and reports are completed. The most tangible impact is in the protocols and guidelines for forward deployed REBOA utilization. The COMPASS device has now been added to the Joint Trauma System Clinical Practice Guideline on REBOA utilization in the operational environment. In addition, the device is being fielded and utilized by a number of forward deployed medical/surgical units, including small austere surgical teams that otherwise would not be able to safely perform REBOA.
Dr. Matthew Martin, AMTI Innovator for this initiative stated, “This work would not have been possible or practical without the generous funding support of AMTI. Validation tests of this nature in large animal models are prohibitively expensive without a dedicated line of funding and other administrative support. The AMTI funding was also critical to the ability to do a complete and thorough series of validation tests starting from uninjured animals and proceeding through to a realistic model of major hemorrhage being managed under austere battlefield conditions.”
This work has supplied adequate data to support clinical use of the COMPASS device in the deployed or operational environment by trained providers. Future iterations plan to evaluate the use of the COMPASS device by less experienced personnel such as basic combat medics/corpsman, the use of the device to accurately guide and perform fine titrations of REBOA with only partial balloon inflation, and to assess any updated/improved versions of this or similar devices.
Dr. Martin added that, “This work represents how immediately impactful and clinically relevant data can be obtained with adequate funding support (via AMTI) and utilizing the great pool of expertise available at our military medical centers. In addition, this was a fantastic example of close collaboration between U.S. Army and U.S. Navy innovators who are guided by the prime mission of improving combat care and survivability for our Service Members.”
For additional information on this AMTI funded project, please contact Ms. Holly Pavliscsak at holly.h.pavliscsak.ctr@mail.mil.
This article was published in the July 2021 issue of the TATRC Times.